Insights from a CSL-sponsored Harris Poll survey†
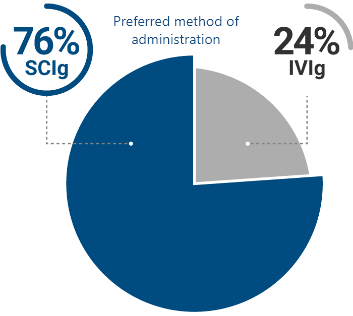
Most CIDP patients surveyed preferred SCIg over IVIg2
Of patients surveyed who have used both IVIg and SCIg, 3 out of 4 preferred SCIg



Hizentra is indicated for maintenance therapy in adults with chronic inflammatory demyelinating polyneuropathy (CIDP) to prevent relapse of neuromuscular disability and impairment.
The EAN/PNS* guideline on diagnosis and treatment of CIDP “strongly recommends” SCIg for CIDP maintenance1
See SCIg highlightsInsights from a CSL-sponsored Harris Poll survey†
Of patients surveyed who have used both IVIg and SCIg, 3 out of 4 preferred SCIg
Hizentra offers greater flexibility to personalize treatment to fit patients’ lives, addressing clinical challenges and lifestyle burdens associated with IVIg. See how Hizentra provides consistent Ig levels and more.
Hizentra Prefilled Syringes (PFS) give your patients
proven protection with a first and only for Ig
Hizentra Prefilled Syringes (PFS) give your patients proven protection with a first and only for Ig
Discover the convenience
Elevating Patients’ Experience
Learn Why All Hizentra Patients Will Be Transitioned From Vials To Prefilled Syringes Learn more
Connect with CSL Behring Medical Affairs to find additional information and ask questions.