Overview
How to
Self-administer Hizentra
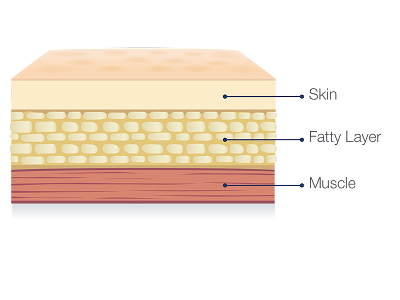
Hizentra is a subcutaneous immunoglobulin, or SCIg, which means it is infused into the fatty layer located just under the skin, and NOT in a vein.

Overview
Hizentra is a subcutaneous immunoglobulin, or SCIg, which means it is infused into the fatty layer located just under the skin, and NOT in a vein.

Prefilled Syringes
Before you can self-infuse Hizentra, you will need proper training from a healthcare professional. Several resources are available here to help you and your doctor make sure you're comfortable with the self-infusion process.
Watch the instruction videos here or download the self-administration guide to review the step-by-step instructions on preparation, proper infusion techniques, and administration.

Infusion Sites
You can use up to 8 infusion sites at the same time. In clinical trials, most patients used 4 or fewer infusion sites. If you are using more than one infusion site, be sure the infusion sites are at least 2 inches apart. Use a different site from the last time you infused. New sites should be at least 1 inch from a previous site.
Discuss any questions you may have about selecting an appropriate infusion site(s) with your doctor.

INFUSION UNDER YOUR SKIN
Hizentra is a subcutaneous Ig (SCIg), infused into the fatty tissue just below the top layer of your skin. Remember, you aren't alone, and Hizentra Connect can send a nurse to your home to help you get confident with self-infusing.

Needle lengths are drawn to scale.
*The needle used for Hizentra infusions is relatively small. Depending on your size and weight, your doctor will recommend the appropriate supplies.

From in-home nurse training visits to financial support and insurance navigation, free help is available so you can live confidently.
To learn more, call 1-877-355-4447 to enroll, Mon–Fri, 8 AM–8 PM ET. Or, you can start your enrollment online today.
WARNING: Thrombosis (blood clots) can occur with immune globulin products, including Hizentra. Risk factors can include: advanced age, prolonged immobilization, a history of blood clotting or hyperviscosity (blood thickness), use of estrogens, installed vascular catheters, and cardiovascular risk factors.
If you are at high risk of blood clots, your doctor will prescribe Hizentra at the minimum dose and infusion rate practicable and will monitor for signs of clotting events and hyperviscosity. Always drink sufficient fluids before infusing Hizentra.
See your doctor for a full explanation, and the full prescribing information for complete boxed warning.
Hizentra®, Immune Globulin Subcutaneous (Human), 20% Liquid, is a prescription medicine used to treat:
Treatment with Hizentra might not be possible if your doctor determines you have hyperprolinemia (too much proline in the blood), or are IgA-deficient with antibodies to IgA and a history of hypersensitivity. Tell your doctor if you have previously had a severe allergic reaction (including anaphylaxis) to the administration of human immune globulin. Tell your doctor right away or go to the emergency room if you have hives, trouble breathing, wheezing, dizziness, or fainting. These could be signs of a bad allergic reaction.
Inform your doctor of any medications you are taking, as well as any medical conditions you may have had, especially if you have a history of diseases related to the heart or blood vessels, or have been immobile for some time. Inform your physician if you are pregnant or nursing, or plan to become pregnant.
Infuse Hizentra under your skin only; do not inject into a blood vessel.
Self-administer Hizentra only after having been taught to do so by your doctor or other healthcare professional, and having received dosing instructions for treating your condition.
Immediately report to your physician any of the following symptoms, which could be signs of serious adverse reactions to Hizentra:
Hizentra is made from human blood. The risk of transmission of infectious agents, including viruses and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent and its variant (vCJD), cannot be completely eliminated.
The most common side effects in the clinical trials for Hizentra include redness, swelling, itching, and/or bruising at the infusion site; headache; chest, joint or back pain; diarrhea; tiredness; cough; rash; itching; fever, nausea, and vomiting. These are not the only side effects possible. Tell your doctor about any side effect that bothers you or does not go away.
Before receiving any vaccine, tell immunizing physician if you have had recent therapy with Hizentra, as effectiveness of the vaccine could be compromised.
Please see full prescribing information for Hizentra, including boxed warning and the patient product information.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
You can also report side effects to CSL Behring's Pharmacovigilance Department at 1-866-915-6958.