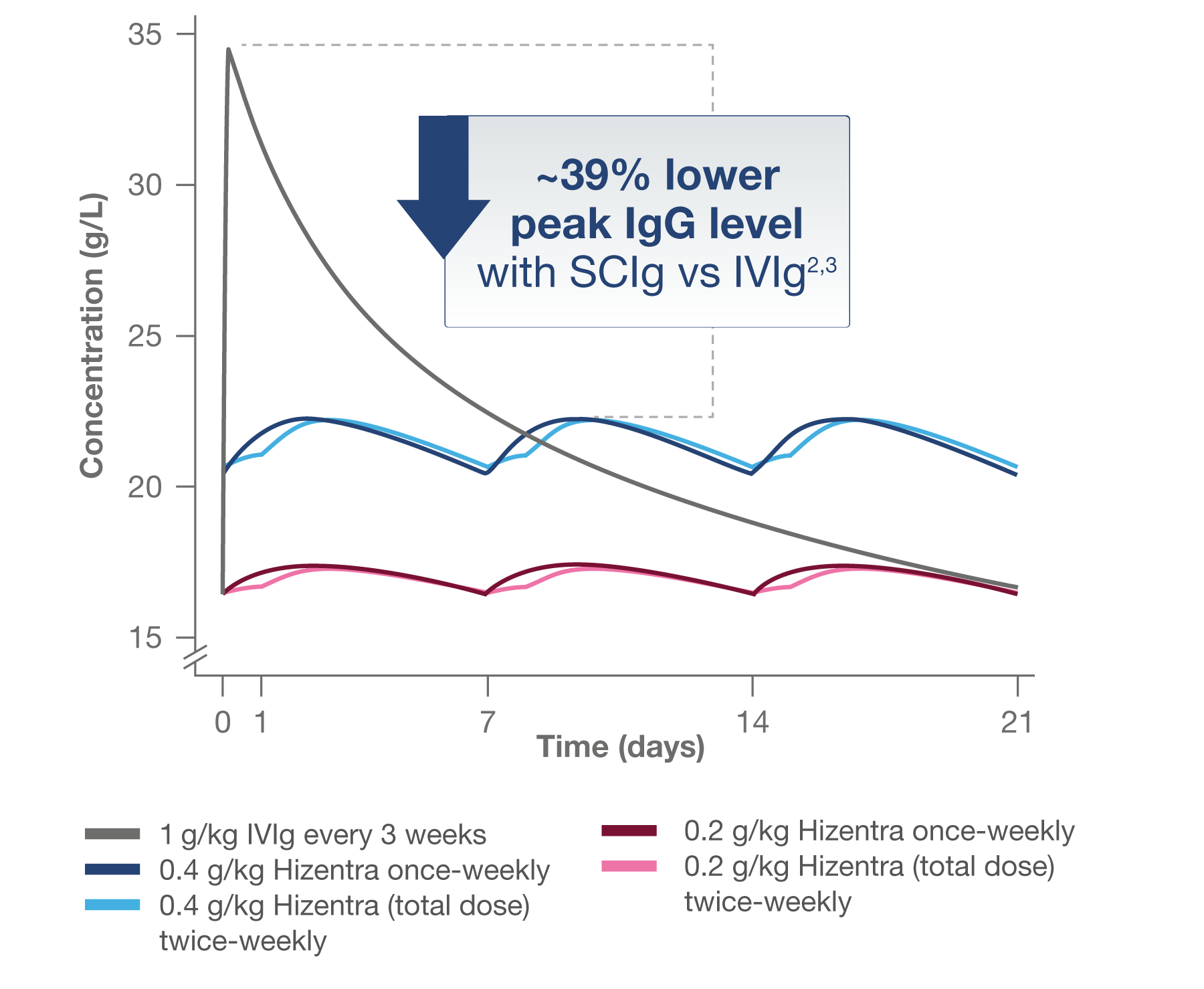
As demonstrated in the pharmacokinetic analysis of the PATH Study
‡PATH Study Design: Data from a randomized, multicenter, double-blind, placebo-controlled, parallel-group, phase III study of 2 doses of weekly Hizentra versus placebo for the treatment of chronic inflammatory demyelinating polyneuropathy (CIDP)—the PATH Study. Subjects who relapsed during the post-randomization phase were to receive IVIg as a rescue medication within 1 week. CIDP relapse was defined as a≥1-point increase in adjusted Inflammatory Neuropathy Cause and Treatment (INCAT) score compared with baseline.
On IVIg, I experienced fatigue leading up to my infusions. With Hizentra, I'm able to maintain stable Ig levels so my strength stays more consistent and I can remain active.
Explore tools and information to help you tailor treatment to individual patient needs
Dosing calculatorSee how Hizentra was proven in the largest Ig study in CIDP and further evaluated in an extension study
Clinical trial dataExplore volumes, rates, and other information to help your patients self-administer Hizentra
Administration guidance