
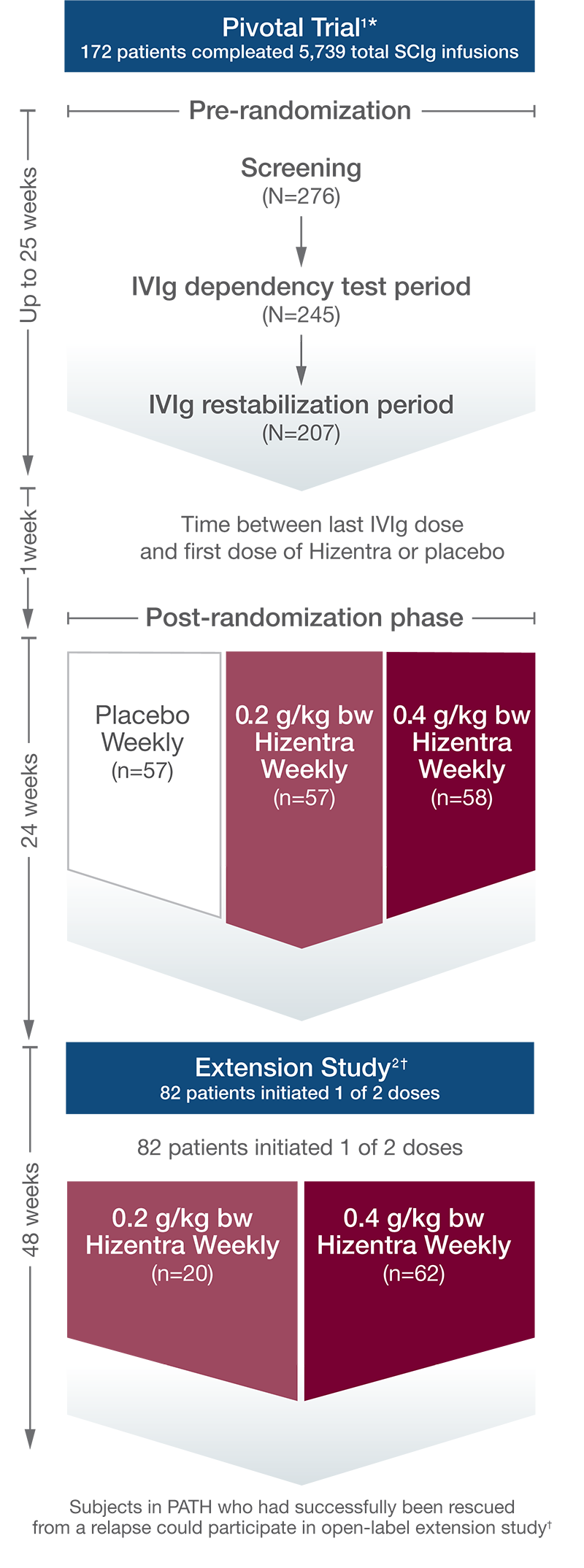
Largest Ig Study in CIDP (n=172)

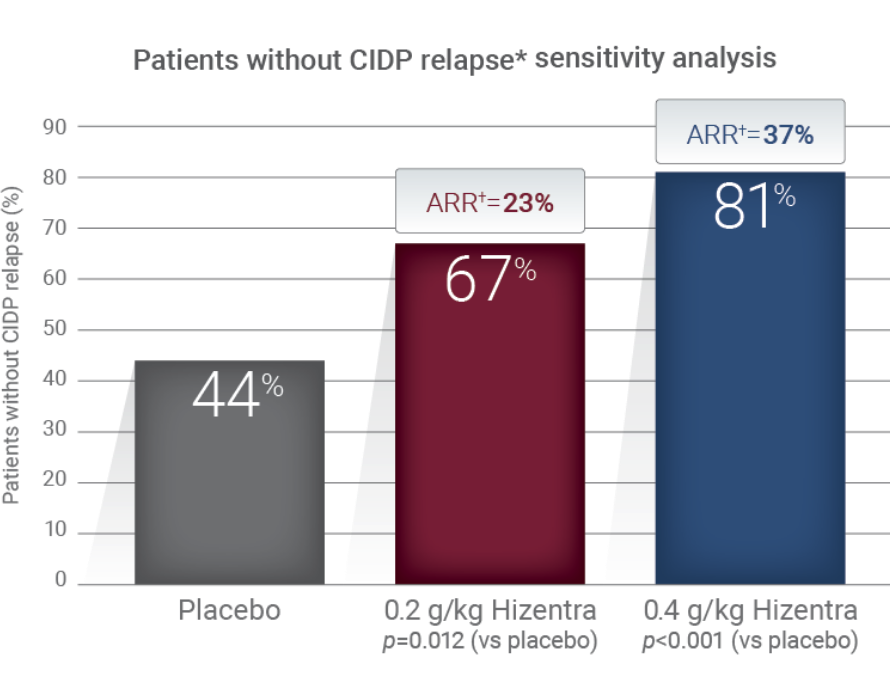
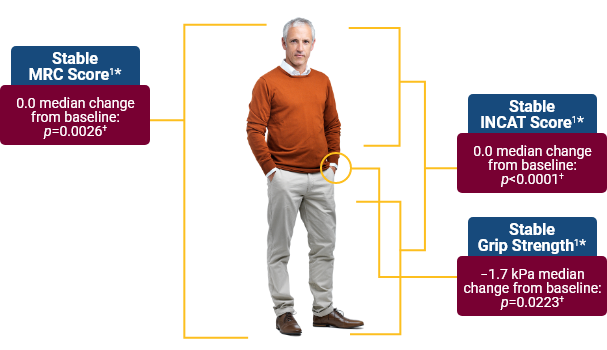
The percentage of CIDP patients who relapsed or withdrew for any other reason during SCIg treatment was significantly lower with Hizentra than with placebo.1*
*
PATH Study Design: Data from a randomized, multicenter, double-blind, placebo-controlled, parallel-group, phase III study of 2 doses of weekly Hizentra versus placebo for the treatment of chronic inflammatory demyelinating polyneuropathy (CIDP)—the PATH Study. Subjects who relapsed during the post-randomization phase were to receive IVIg as a rescue medication within 1 week. CIDP relapse was defined as a ≥1-point increase in adjusted Inflammatory Neuropathy Cause and Treatment (INCAT) score compared with baseline.
†
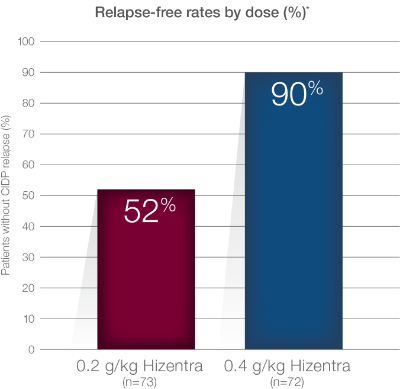
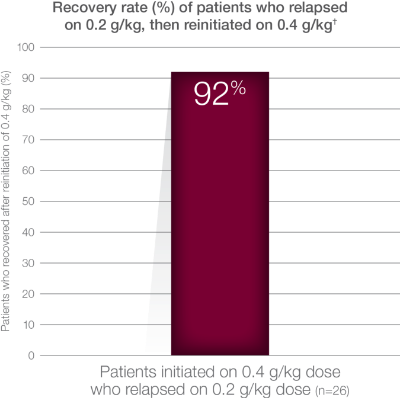
Extension Study Design: 48-week, open-label, prospective extension study to PATH. 82 patients were enrolled: 62 patients were started on 0.4 g/kg weekly infusion of Hizentra, and 20 patients were started on 0.2 g/kg weekly. If clinically stable, patients on 0.4 g/kg were switched to 0.2 g/kg after 24 weeks. Upon CIDP relapse on 0.2 g/kg dose, 0.4 g/kg was either initiated or reinitiated. CIDP relapse defined as a ≥1-point increase in adjusted Inflammatory Neuropathy Cause and Treatment (INCAT) score compared with baseline.
ZERO cases of thrombosis or hemolysis were observed in Hizentra patients in the PATH Study.1 Thrombosis or hemolysis may occur with Ig products, including Hizentra. Please see the boxed warning and section 5 of the full prescribing information for more details.
PATH Extension Study tolerability was similar to the PATH Study. The most frequent ARs in the PATH Extension Study were local infusion site reactions, which occurred more frequently in subjects who received the 0.4 g/kg dose than in subjects who received the 0.2 g/kg dose (18.1% and 9.6%, respectively).
| Placebo (n=57) |
0.2 g/kg Hizentra (n=57) |
0.4 g/kg Hizentra (n=58) |
|
|---|---|---|---|
| Local Reactions‡ | 7.0% | 19.3% | 29.3% |
| Headache | 3.5% | 7.0% | 6.9% |
| Nasopharyngitis | 1.8% | 7.0% | 3.4% |
| Fatigue | 1.8% | 8.8% | 0.0% |
| Upper Respiratory Tract Infection | 3.5% | 5.3% | 3.4% |
| Fall | 0.0% | 5.3% | 1.7% |
| Back Pain | 1.8% | 5.3% | 1.7% |
| Arthralgia | 1.8% | 5.3% | 1.7% |
| Pain in Extremity | 0.0% | 1.8% | 5.2% |

Mild = does not interfere with routine activities.
Moderate = interferes somewhat with routine activities.
Severe = impossible to perform routine activities.

Explore tools and information to help you tailor treatment to individual patient needs
Dosing calculatorExplore volumes, rates, and other information to help your patients self-administer Hizentra
Administration guidance